fekitari Zvitengeswa zveEUR muStock CAS 1451-83-8 2-Bromo-3-Methylpropiophenone 2-Bromo-1- (3-methylphenyl) Propan-1-One/236117-38-7/59774-06-0/1009- 14-9/705-60-2
fekitari Zvitengeswa zveEUR muStock CAS 1451-83-8 2-Bromo-3-Methylpropiophenone 2-Bromo-1- (3-methylphenyl) Propan-1-One/236117-38-7/59774-06-0/1009- 14-9/705-60-2
Kambani yedu inosimbisa manejimendi, kuunzwa kwevashandi vane tarenda, uye kuvakwa kwevashandi vekuvaka, kuyedza nesimba kuvandudza hunhu uye kuziva mutoro wevashandi.Kambani yedu yakabudirira kuwana IS9001 Certification uye European CE Certification yefekitori Zvitengesi zveEUR muStock CAS 1451-83-8 2-Bromo-3-Methylpropiophenone 2-Bromo-1- (3-methylphenyl) Propan-1-One/236117-38 -7/59774-06-0/1009-14-9/705-60-2, Tinokukurudzira kuti ubate sezvo tanga tichida shamwari mukati mekambani yedu.Isu tine chokwadi chekuti uchafumura kuita kambani nesu kwete kungobereka zvibereko asiwo kunobatsira.Isu takagadzirira kukupa iwe zvaunoda.
Kambani yedu inosimbisa manejimendi, kuunzwa kwevashandi vane tarenda, uye kuvakwa kwevashandi vekuvaka, kuyedza nesimba kuvandudza hunhu uye kuziva mutoro wevashandi.Kambani yedu yakabudirira kuwana IS9001 Certification uye European CE Certification yeChina 1451 83 8 uye CAS 1451 83 8, Mazuva ano zvigadzirwa zvedu zvinotengeswa kwese kwese kudzimba nekune dzimwe nyika kuvonga nerutsigiro rwenguva dzose uye rutsva rwevatengi.Isu tinopa chemhando yepamusoro chigadzirwa uye mutengo wemakwikwi, gamuchirai vatengi venguva dzose uye vatsva vanoshandira pamwe nesu!
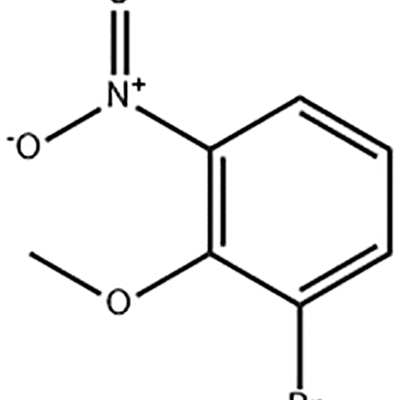
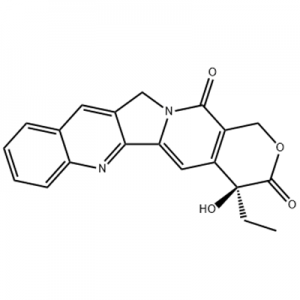
1-Bromo-2-methoxy-3-nitro-benzene inoshandiswa sepakati peEltrombopag .
Eltrombopag, yakagadziridzwa naGlaxoSmithKline (GSK) kuUK uye yakazogadziriswa pamwe chete neNovartis kuSwitzerland, ndiyo yekutanga uye yakatenderwa chete mamorekuru madiki asiri peptide TPO receptor agonist munyika.Eltrombopag yakabvumidzwa neUS FDA muna 2008 kurapwa kweidiopathic thrombocytopenic purpura (ITP), uye muna 2014 kurapwa kweaplastic anemia (AA).Ndiwo zvakare mushonga wekutanga wakabvumidzwa neUS FDA kurapwa kweAA mumakore makumi matatu apfuura.
Muna Zvita 2012, US FDA yakabvumidza Eltrombopag kurapwa kwethrombocytopenia muvarwere vane chisingaperi hepatitis C (CHC), kuitira kuti varwere veHepatitis C vane hurombo husina kunaka nekuda kwekuderera kweplatelet count vanogona kutanga nekuchengetedza interferon based standard therapy yezvirwere zvechiropa.Musi waFebruary 3, 2014, GlaxoSmithKline yakazivisa kuti FDA yakapa mukana wekurapa mushonga weEltrombopag kurapwa kwehemopenia muvarwere vane yakanyanya chemicalbook aplastic anemia (SAA) vasina kupindura zvizere kune immunotherapy.Musi waNyamavhuvhu 24, 2015, US FDA yakabvumidza Eltrombopag kurapwa kwethrombocytopenia muvakuru nevana vane makore 1 uye kupfuura vane chronic immune thrombocytopenia (ITP) vasina mhinduro yakakwana kune corticosteroids, immunoglobulins kana splenectomy.Musi waJanuary4, 2018, Eltrombopag yakabvumidzwa kunyorwa muChina kurapwa kwekutanga immune thrombocytopenia (ITP).
Kambani yedu inosimbisa manejimendi, kuunzwa kwevashandi vane tarenda, uye kuvakwa kwevashandi vekuvaka, kuyedza nesimba kuvandudza hunhu uye kuziva mutoro wevashandi.Kambani yedu yakabudirira kuwana IS9001 Certification uye European CE Certification yefekitori Zvitengesi zveEUR muStock CAS 1451-83-8 2-Bromo-3-Methylpropiophenone 2-Bromo-1- (3-methylphenyl) Propan-1-One/236117-38 -7/59774-06-0/1009-14-9/705-60-2, Tinokukurudzira kuti ubate sezvo tanga tichida shamwari mukati mekambani yedu.Isu tine chokwadi chekuti uchafumura kuita kambani nesu kwete kungobereka zvibereko asiwo kunobatsira.Isu takagadzirira kukupa iwe zvaunoda.
factory Zvitengesi zveChina 1451 83 8 uye CAS 1451 83 8, Mazuva ano zvigadzirwa zvedu zvinotengeswa kwese kwese kudzimba nekune dzimwe nyika kuvonga nerutsigiro rwenguva dzose uye rutsva rwevatengi.Isu tinopa chemhando yepamusoro chigadzirwa uye mutengo wemakwikwi, gamuchirai vatengi venguva dzose uye vatsva vanoshandira pamwe nesu!